25
2022
-
03
Effects of trace elements chelated with oligosaccharides on production performance, trace element metabolism and antioxidant activity in broilers
Wang Xin¹, Yang Zaibin¹, Zhou Jianqun², Li Dandan¹, Li Pengfei¹, Liu Kaihong¹, Zhang Yinfeng¹, Yang Weiren¹, Jiang Shuzhen¹* (1. Shandong Agricultural University
(1College of Animal Science and Technology, Shandong Agricultural University, Key Laboratory of Animal Bioengineering and Disease Prevention of Shandong Province, Tai'an, 271018; 2Nanning Zewell Feed Co., Ltd., Nanning, 530221)
Abstract: The aim of this study was to investigate the effects of oligosaccharide chelated trace elements in diet on the production performance, trace element contents in blood and liver, and antioxidant properties of broiler chickens. Six hundred healthy one-day-old AA commercial broilers were selected and randomly divided into six treatments, with five replicates in each treatment. Treatment 1 was inorganic trace element treatment (IE-100), in which copper, zinc, manganese, iron, iodine and selenium were supplemented in the form of inorganic trace element premixes (CuSO4·5H2O, ZnSO4·H2O, MnSO4·H2O, FeSO4·H2O, KIO3, Na2SeO3), and the addition amounts were 8, 70, 90, 50, 0.5 and 0.3 mg/kg, respectively. Treatments 2, 3, 4 and 5 were organic composite trace element treatments, in which oligosaccharide chelated trace element premixes (oligosaccharide chelated copper, zinc, manganese and iron; KIO3 and Na2SeO3) were used to supplement the feed, and the element levels were 100% (OE-100), 75% (OE-75), 50% (OE-50) and 25% (OE-25) of treatment 1, respectively. Treatment 6 was no trace element addition (OE-0). The experiment was divided into two stages (1-21 d and 22-42 d). The weighing and slaughtering experiments were carried out at 21 d and 42 d, and the production performance, trace element content and antioxidant properties were determined. The results showed that: 1) Compared with IE-100, OE-100 significantly increased (P<0.05) the ADG (1-42 d), blood and liver iron (21 d), serum and liver Cu-Zn SOD (42 d) activities of broilers, and significantly reduced the liver MDA (42 d) content (P<0.05). 2) Compared with OE-0, ADG (22-42 d and 1-42 d) of OE-75 broilers was significantly increased (P<0.05), and F/G (22-42 d and 1-42 d) was significantly decreased (P<0.05). IE-100, OE-100, OE-75, OE-50 and OE-25 significantly increased (P<0.05) the iron (21 d), zinc and copper (42 d) in blood and liver of broilers. The activities of Cu-Zn SOD and GSH-Px (21 d and 42 d) in serum and liver of IE-100, OE-100, OE-75 and OE-50 were significantly increased (P<0.05), and the content of MDA (42 d) was significantly decreased (P<0.05). 3) With the decrease of the level of oligosaccharide chelated trace element premix, broiler ADG (1-21 d, 1-42 d and 22-42 d), blood trace elements [iron (21 d), zinc (21 d and 42 d), copper (21 d and 42 d) and manganese (42 d)], liver trace elements [iron and zinc (21 d and 42 d), copper and manganese (42 d)], serum and liver Cu-Zn SOD and GSH-Px activities (21 d and 42 d) decreased in a linear and quadratic manner (P<0.05),
while F/G (22-42 d and 1-42 d), serum (21 d and 42 d) and liver (42 d) MDA content increased in a linear and quadratic manner (P<0.05). The results showed that the optimal levels of oligosaccharide chelated trace elements to improve broiler production performance, element (copper, zinc, manganese and iron) serum and liver reserves, serum and liver antioxidant performance were 25%, 50% and 75%, respectively, and the level equivalent to the comprehensive performance of inorganic trace elements was 50%-75%.
Keywords: oligosaccharide chelated trace elements; growth performance; trace element content; antioxidant performance
Chinese Library Classification Number: S816.72 Document Identification Code: Article Number:
Effects of Oligosaccharide-chelated Trace Elements on Growth Performance, Trace Element Metabolism and Antioxidant Properties of Broilers
WANG Xin1,YANG Zai-bin1,ZHOU Jian-qun2,LI Dan-dan1,LI Peng-fei1,LIU Kai-hong1,ZHANG Yin-feng1,YANG Wei-ren1,JIANG Shu-zhen1,* (1.Shandong Provincial Key Laboratory of Animal Biotechnology and Disease Control and Prevention,College of Animal Science and Technology, Shandong Agricultural University,Tai 'an 271018, China;2. Nanning Zewei Feed Co., Ltd,Nanning 530221,China)
Abstract:The aim of this experiment was to study the effects of different oligosaccharide-chelated trace elements level on growth performance, trace element content and antioxidant properties in blood and liver of broilers. 600 healthy one-day-old AA commercial broilers were randomly divided into 6 treatments with 5 replicates for each treatment. Treatment 1 was inorganic trace elements (IE-100). The inorganic trace elements premix (CuSO4·5H2O, ZnSO4·H2O, MnSO4·H2O, FeSO4·H2O, KIO3, Na2SeO3) was used to supplement copper, zinc, manganese, iron, iodine and selenium with the content of 8, 70, 90, 50, 0.5 and 0.3 mg/kg in the IE-100, respectively. Treatment 2, 3, 4 and 5 were organic composite trace elements, and the diets were IE-100 in which inorganic trace elements were replaced by oligosaccharide chelated trace element premix (oligosaccharide chelated Cu, Zn, Mn and Fe, KIO3 and Na2SeO3) at the level of 100%(OE-100) 75%(OE-75) 50%(OE-50) and 25%(OE-25), respectively. Treatment 6 was no additional trace elements (OE-0). The experiment was divided to two stages (1-21 d and 22-42 d). Weighing and slaughter experiments were conducted on 21 d and 42 d to determine the production performance, trace element content and antioxidant properties. 1) Results showed that OE-100 increased (P<0.05) ADG (1~42 d) of broiler, the activity of blood and liver iron (21 d), serum and liver Cu-Zn SOD (42 d), and the content of liver MDA (42 d) decreased (P<0.05), compared with IE-100. 2) Compared with OE-0, ADG (22-42 d and 1-42 d) of OE-75 broilers increased (P<0.05), and F/G (22-42 d and 1-42 d) decreased (P<0.05); IE-100, OE-100, OE-75, OE-50 and OE-25 increased (P<0.05) iron (21 d), zinc and copper (42 d) in blood and liver of broilers (P<0.05); Serum and liver Cu-Zn SOD (21 d), GSH-Px (42 d) activities of IE-100, OE-100, OE-75 and OE-50 increased (P<0.05), MDA (42 d) content decreased (P<0.05). 3) With the decreasing of oligosaccharide chelated trace element, the ADG (1-21 d, 1-42 d and 22-42 d), trace elements in blood [iron (21 d), zinc and copper (21 d and 42 d), and manganese (42 d)], trace elements in liver [iron and zinc (21 d and 42 d), copper and manganese (42 d)], serum and liver Cu-Zn SOD and GSH-Px (21 d and 42 d) decreased linearly and quadratically (P<0.05), while F/G (22-42 d and 1-42 d) and the content of MDA in serum (21 d and 42 d) and liver (42 d) increased linearly and quadratically (P<0.05). In conclusion, the optimum levels of oligosaccharide chelated trace elements in improving broiler performance, serum and liver accumulation of copper, zinc, manganese and iron, serum and liver antioxidant capacity were 25%, 50% and 75%, respectively, and the equivalent level of oligosaccharide chelated trace elements to inorganic trace elements according to comprehensive index are 50%-75%.
Key words: oligosaccharide-chelated trace elements; growth performance; trace element accumulation; antioxidant properties
Trace elements are important nutrients for animal growth and development, and are also important components of many enzymes in animals. They play a very important role in animal metabolism and healthy development [1,2]. Compared with traditional inorganic trace elements, organic trace elements have the advantages of high biological availability and reduced environmental pollution [3-7]. Studies have shown that copper-chitosan nanoparticles (100 mg/kg) can improve the growth performance of broilers [8], and hydroxymethionine chelated zinc (60 mg/kg) can improve the growth performance of broilers aged 1-6 weeks [9]. Oligosaccharide chelated trace elements prepared by chelating sugarcane molasses with copper sulfate, zinc, manganese, and iron can meet the needs of broilers for a variety of trace elements and can replace inorganic trace elements. They are of high purity, good stability, and safe and pollution-free [10]. However, there are few reports on the effects of oligodisaccharide chelated trace elements on broiler growth performance, trace element content in blood and liver, and antioxidant activity. This study aims to explore the effects of gradient reduction of oligodisaccharide chelated trace element levels on broiler growth performance, trace element content in blood and liver, and antioxidant activity, and to provide a scientific basis for reducing the use of trace elements in broiler feed.
1 Materials and methods
1.1 Experimental materials and experimental design
1.1.1 Experimental materials 1‰ inorganic trace elements (IE) premix: copper (CuSO4·5H2O, feed grade, 98.5%), zinc (ZnSO4·H2O, feed grade, 98.5%), manganese (MnSO4·H2O, feed grade, 99.0%), iron (FeSO4·H2O, feed grade. 98.5%), iodine (KIO3, feed grade, 99.0%), selenium (Na2SeO3, feed grade, 98.5%), the contents were 8, 70, 90, 50, 0.5 and 0.3 g/kg respectively, and the diluent was zeolite powder, produced by Taian Taihang Mineral Feed Premix Co., Ltd.
Oligosaccharides chelated trace elements (OE) premix: It is composed of oligosaccharides chelated trace elements (copper, zinc, manganese and iron), KIO3 (feed grade, 99.0%) and Na2SeO3 (feed grade, 98.5%). Its copper, zinc, manganese, iron, iodine and selenium content is the same as 1‰ inorganic trace element premix, provided by Nanning Zeweier Feed Co., Ltd.
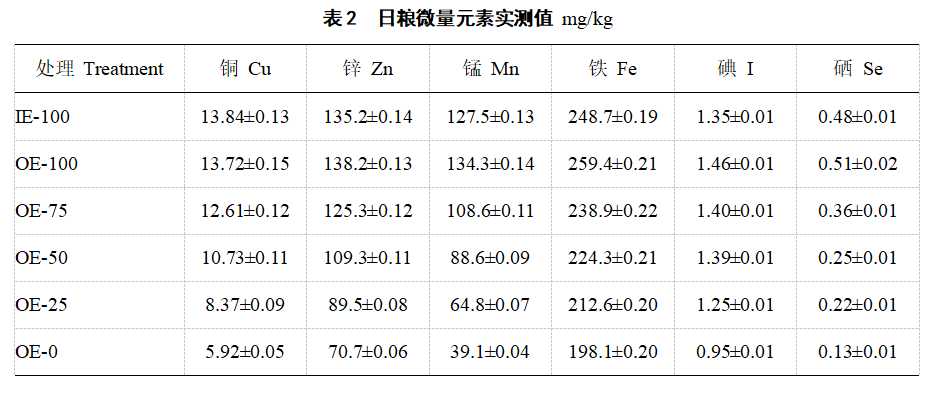
1.1.2 Experimental animals, diets and experimental design 600 healthy one-day-old AA commercial broilers were selected and randomly divided into 6 treatments, each with 5 replicates and 20 chickens in each replicate. There was no significant difference in initial body weight among the treatments (P>0.05). Treatment 1 was an inorganic trace element (IE-100) treatment, in which a homemade 1‰ inorganic trace element premix was added, and the addition of copper, zinc, manganese, iron, iodine and selenium in the diet was 8, 70, 90, 50, 0.5 and 0.3 mg/kg, respectively. Treatments 2, 3, 4 and 5 were organic composite trace element treatments, in which oligosaccharide chelated trace element premixes (oligosaccharide chelated copper, zinc, manganese and iron; KIO3 and Na2SeO3) were added, so that the element levels in the diet were 100% (OE-100), 75% (OE-75), 50% (OE-50) and 25% (OE-25) of treatment 1, respectively. Treatment 6 was a treatment without trace element addition (OE-0). The experiment was divided into two stages (1-21 d and 22-42 d), and its basic diet and nutrient level were prepared according to the recommended standards of NRC (2012) (Table 1). The measured values of trace element levels in the feed are shown in Table 2.
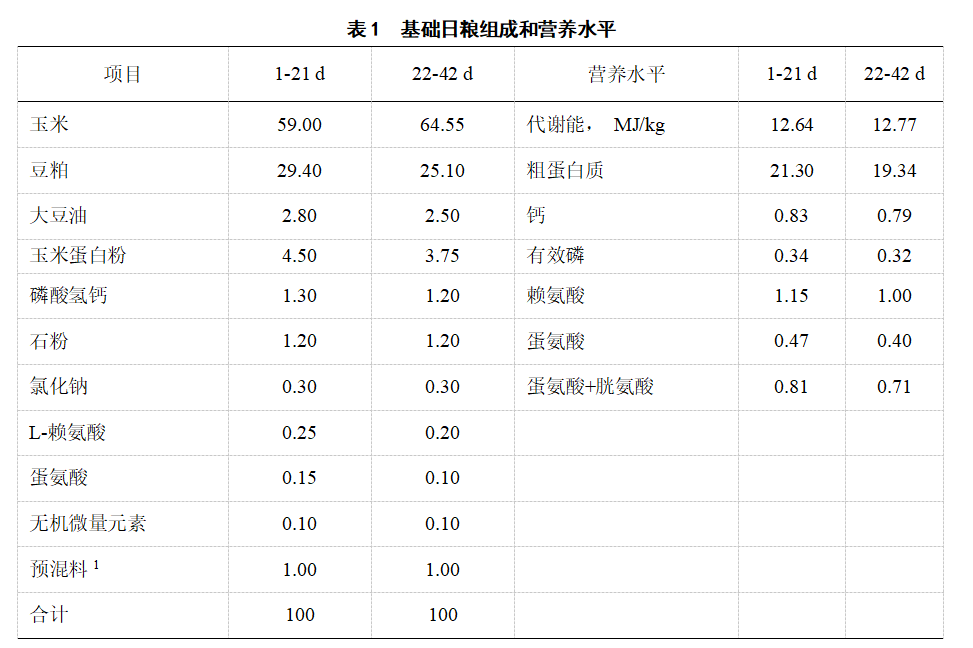
The premix provides per kilogram of diet: VA 11,500 IU; VD3 3,500 IU; VE 30 mg; VK3 3 mg; VB1 3.38 mg; VB2 9.00 mg; VB6 8.96 mg; VB12 0.025 mg; choline chloride 800 mg; calcium pantothenate 13 mg; niacin 45 mg; biotin 0.08 mg; folic acid 1.20 mg.
1.1.3 Feeding and management Broilers were raised in cages in the same chicken house. The chicken house was thoroughly cleaned and disinfected 24 hours before the chickens were introduced, and the room temperature was raised to 35-36°C. During the experiment, the broilers were ensured to have continuous light, natural ventilation, free access to food and water, and the chicken house was cleaned every day and immunized routinely.
1.2 Measurement indicators and methods
1.2.1 Production performance During the experiment, the feed intake was recorded every day and weighed in replicates at 1, 21 and 42 days of the experiment. The average daily feed intake (ADFI), average daily weight gain (ADG) and feed-to-weight ratio (F/G) were calculated.
1.2.2 Collection and processing of blood samples On the 21st and 42nd days of the experiment, two chickens were randomly selected for each replicate and fasted for 12 hours. 10 mL of blood was collected aseptically from the wing root vein using a vacuum anticoagulant tube, and 10 mL of blood was collected aseptically using a vacuum procoagulant tube. The anticoagulated blood was stored in a -20 ℃ refrigerator for the detection of the storage of trace elements copper, manganese, zinc, and iron in the blood. The procoagulant blood was placed in warm water at an angle for 30 minutes, centrifuged (2000 r/min) for 10 minutes to separate the serum, and the serum was packaged and numbered and stored at -20 ℃ for the detection of serum antioxidant enzymes and malondialdehyde content.
1.2.3 Slaughter test and collection of liver samples On the 21st and 42nd days of the experiment, 2 chickens were randomly selected from each replicate and fasted for 24 hours. They were bled and slaughtered, and the abdominal cavity was opened to quickly take out about 100 g of liver and store it in a self-sealing bag at -20℃ for the detection of the storage of trace elements copper, manganese, zinc and iron in the liver; about 10 g of liver was taken and stored in a cryopreservation tube at -20℃ for the detection of liver antioxidant enzymes and malondialdehyde content.
1.2.4 Determination of trace element storage The storage of trace elements copper, manganese and zinc in blood and liver was determined by inductively coupled plasma mass spectrometry (GB 2009.268-2016), and the storage of trace element iron was determined by flame atomic absorption spectrometry (GB 2009.90-2016).
1.2.5 Determination of serum antioxidant enzymes and malondialdehyde content Cu-Zn superoxide dismutase (Cu-Zn SOD) was determined according to the xanthine oxidase method (hydroxylamine method), and glutathione peroxidase (GSH-Px) and malondialdehyde (MDA) were determined according to the chemical colorimetric method. Cu-Zn SOD (A001-1), GSH-Px (A005) and MDA (A003-1) test kits were purchased from Nanjing Jiancheng Bioengineering Institute.
1.2.6 Determination of liver antioxidant enzymes and malondialdehyde content After thawing the liver sample at room temperature, take an appropriate amount, make a 10% homogenate under ice bath conditions, centrifuge (3 000 r/min) for 10 min, and then take the supernatant to detect antioxidant enzyme activity and malondialdehyde content. The determination method and kit are the same as 1.2.5.
1.3 Data statistics The data were processed by Excel and analyzed by SAS 9.2 statistical software for one-way ANOVA. Ducan’s multiple comparison was used to process the mean values. P < 0.05 was considered significant, and P < 0.01 was considered extremely significant. Linear regression was used to analyze the linear and quadratic relationships between production performance, trace element content and other indicators under different addition levels of organic trace elements.
2 Results and analysis
2.1 Production performance Compared with IE-100, OE-100 significantly increased the ADG of broilers (1-42 d) (P < 0.05, Table 3). Compared with OE-0, the ADG (22-42 d and 1-42 d) of OE-75 broilers was significantly increased (P < 0.05), and the F/G (22-42 d and 1-42 d) was significantly reduced (P < 0.05). The ADG of broilers (1-21 d, 1-42 d and 22-42 d) decreased linearly (P<0.05) with the decrease of the level of oligosaccharide chelated trace element premix, while the F/G (22-42 d and 1-42 d) increased linearly (P<0.05).
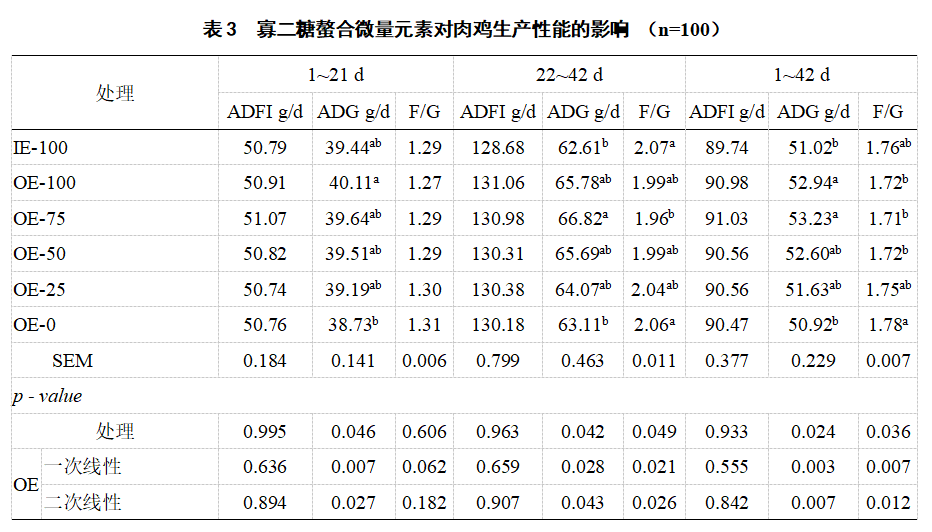
ADFI, average daily feed intake; ADG, average daily feed intake; F/G, feed-to-weight ratio. Data in the same column with different letters indicate significant differences (P<0.05), the same below.
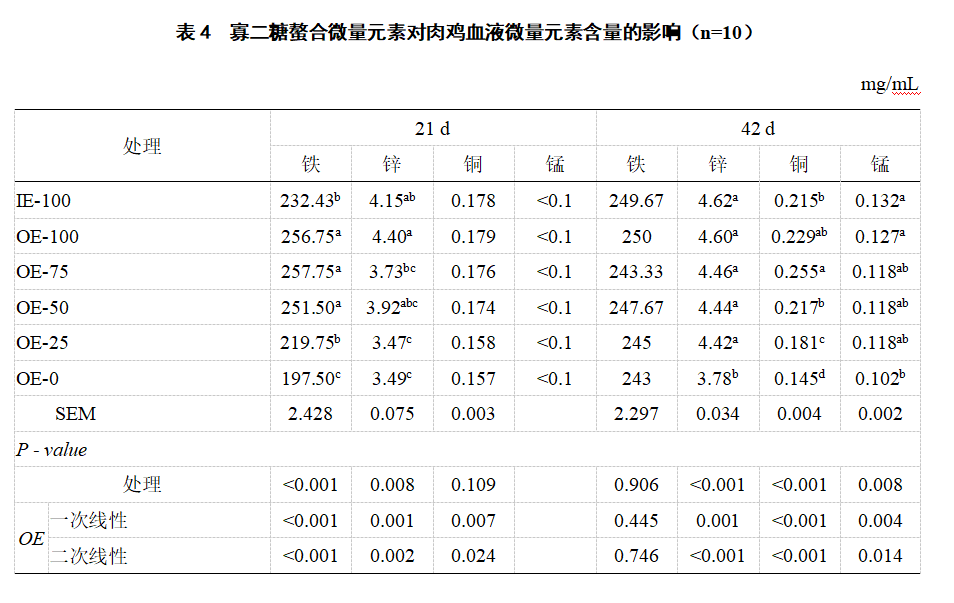
2.2 Blood iron, copper, zinc and manganese levels Compared with IE-100, OE-100 significantly increased blood iron (21 d) in broilers (P<0.05, Table 4). Compared with OE-0, blood iron (21 d), zinc and copper (42 d) in IE-100, OE-100, OE-75, OE-50 and OE-25 were significantly increased (P<0.05). As the level of oligosaccharide chelated trace element premix decreased, the levels of iron (21 days), zinc (21 days and 42 days), copper (21 days and 42 days) and manganese (42 days) in broiler blood decreased linearly (P<0.05). In this study, the manganese content in blood was below the detection limit at 21 days.
2.3 Storage of iron, copper, zinc and manganese in the liver Compared with IE-100, OE-100 significantly increased the iron content of broiler liver (21 days) (P<0.05, Table 5). Compared with OE-0, IE-100, OE-100, OE-75, OE-50 and OE-25 significantly increased the iron content of broiler liver at 21 days and the iron, zinc, copper and manganese at 42 days (P<0.05). As the level of oligosaccharide chelated trace element premix decreased, the storage of iron and zinc (21 days and 42 days), copper and manganese (42 days) in broiler liver showed a linear decrease (P<0.05) in the first and second order.
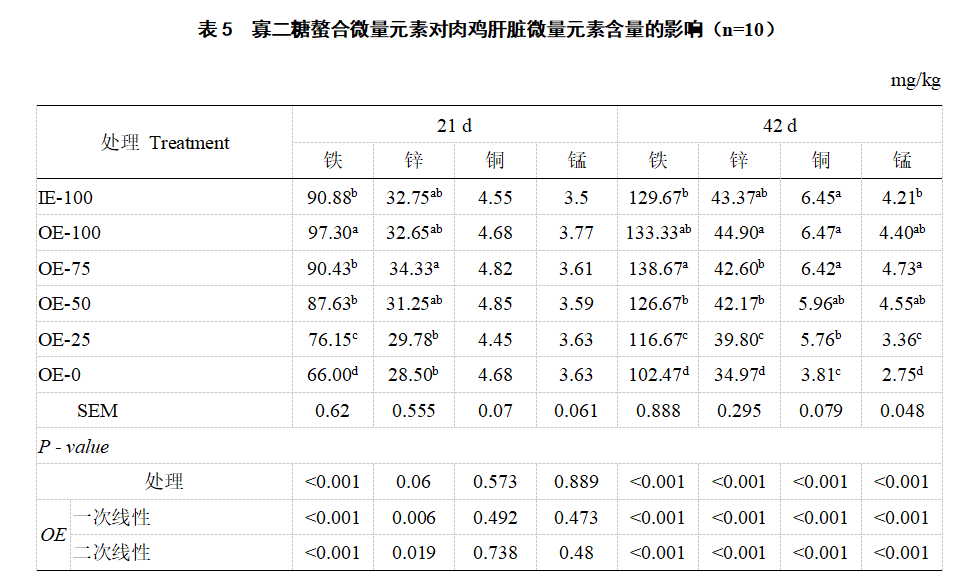
2.4 Serum antioxidant enzyme activity and malondialdehyde content Compared with IE-100, OE-100 significantly increased the serum Cu-Zn SOD (42 d) activity of broiler chickens (P<0.05, Table 6). Compared with OE-0, the serum Cu-Zn SOD and GSH-Px (21 d and 42 d) activities of IE-100, OE-100, OE-75, OE-50 and OE-25 were significantly increased (P<0.05), and the MDA (21 d and 42 d) content was significantly decreased (P<0.05). The serum Cu-Zn SOD and GSH-Px activities (21 d and 42 d) decreased linearly in a first and second order manner with the decrease of the oligosaccharide chelated trace element premix level (P<0.05), while the MDA (21 d and 42 d) content increased linearly in a first and second order manner (P<0.05).
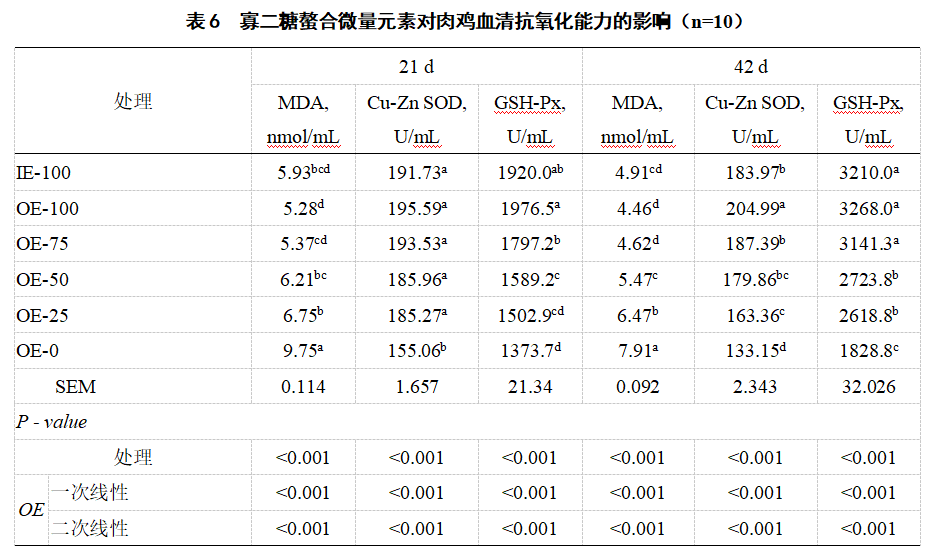
MDA, malondialdehyde; Cu-Zn SOD, Cu-Zn superoxide dismutase; GSH-Px, glutathione peroxidase. Same as the following table.
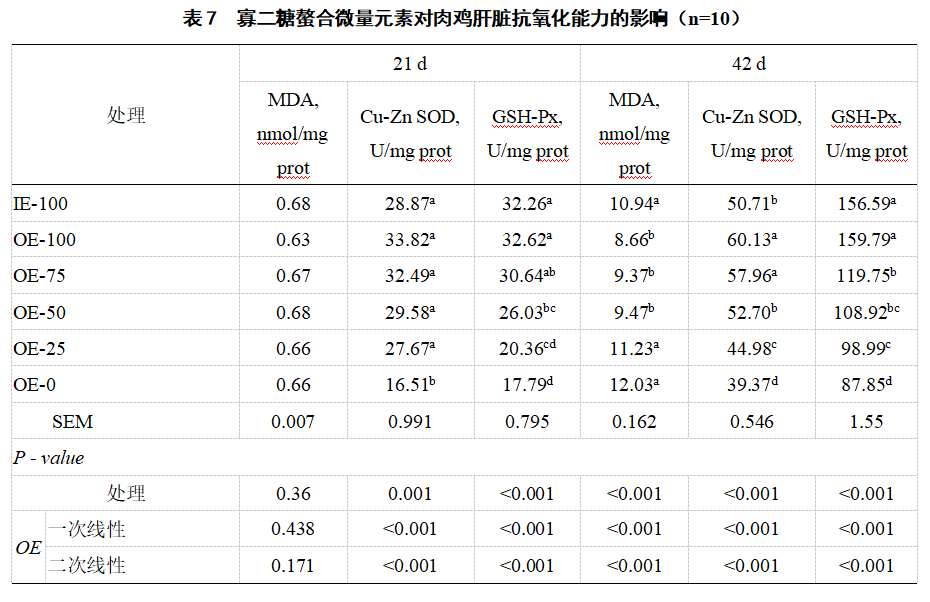
2.5 Antioxidant enzyme activity and malondialdehyde content in liver Compared with IE-100, OE-100 significantly increased the activity of Cu-Zn SOD (42 d) in broiler liver (P<0.05, Table 7), and significantly decreased the content of MDA (42 d) (P<0.05). Compared with OE-0, the activities of Cu-Zn SOD and GSH-Px (21 d and 42 d) in the liver of IE-100, OE-100, OE-75 and OE-50 were significantly increased (P<0.05), and the content of MDA (42 d) was significantly decreased (P<0.05). As the level of oligosaccharide chelated trace element premix decreased, the activities of Cu-Zn SOD and GSH-Px in broiler liver (21 d and 42 d) decreased in a linear manner (P<0.05), while the MDA content (42 d) increased in a linear manner (P<0.05).
3 Discussion
3.1 Growth performance Adding organic trace elements to the diet can promote the growth performance of animals. Studies have shown that replacing 75% of the corresponding inorganic iron, copper, zinc, and manganese with 37.5% methionine chelated iron, copper, zinc, and manganese (1 mg chelated element replaces 2 mg inorganic element) significantly increased broiler ADG and reduced feed-to-weight ratio[11]. Adding pectin oligosaccharide chelated zinc (600 mg/kg) to broiler diets significantly increased ADG and ADFI[12]. Guo Jianlai et al.[13] showed that replacing all the corresponding inorganic trace elements with protein chelated iron, zinc, copper, and manganese significantly increased the daily weight gain of ‘Du×Chang×Da’ three-way piglets, and the feed-to-weight ratio also decreased. CREECH et al.[14] showed that replacing 50% of sulfate with protein chelated copper, zinc, manganese, and iron in the diet also significantly increased daily weight gain in weaned sows. In this experiment, the production performance of broiler chickens was significantly improved by replacing all inorganic trace elements with 75% or 100% oligodisaccharide chelated trace element premix. However, there was no significant difference in the production performance of broiler chickens when 25% oligodisaccharide chelated trace element premix was used to replace all inorganic trace elements. This indicates that 25% oligodisaccharide chelated multi-element trace element premix meets the trace element needs of AA broiler chickens for production performance. The possible reason is that the chelation rate of oligodisaccharide chelated multi-element trace elements is high. After entering the intestine, the trace elements do not exist in the form of ions, but are absorbed in the form of oligodisaccharide chelates. Since the molecules of oligodisaccharide chelates are small, they can be better absorbed into the blood [15]. At the same time, oligodisaccharide chelated multi-element trace elements are lipophilic and can pass through the brush border of the small intestinal villi, be absorbed into the blood through the amino acid and small peptide pathways, and can also enter the blood through glycolipids, glycoproteins and oligosaccharide pathways [16,17]. Trace elements are components of enzyme structure and active center[18]. They promote the metabolism of three major organic substances in the body through various enzymes, thereby improving the production performance of animals. However, some studies have shown that the use of different levels (25, 50, 75, 100 mg/kg) of protein chelated zinc has no significant effect on the growth performance of weaned piglets[19]. This may be because different organic trace elements have different biological valences, production processes and main mechanisms of action. At the same time, different animals have different requirements for trace elements at different stages[10,15]. Within a certain dosage range, trace elements improve the immunity and antioxidant capacity of animals, but may not significantly improve production performance. However, its mechanism needs further confirmation.
3.2 Trace element content The content of trace elements in the blood reflects the body's absorption and utilization of trace elements[20,21]. After circulating in the blood, the main deposition site is the liver[22]. Sun Qiujuan et al.[23] found that using equal amounts of hydroxymethionine to chelate copper, manganese and zinc instead of inorganic salts significantly increased the content of copper, manganese and zinc in the liver of laying hens. SEO et al. [24] found that as the amount of methionine chelated iron added to broiler diets increased, the iron content in the liver increased significantly, and the iron content in the liver reached its highest level when the addition amount was 200 mg. In a pig study, it was found that the addition of 170 mg/kg hydroxymethionine copper to the diet of weaned piglets significantly increased the copper content in the liver [25]. Replacing an equal amount of the corresponding inorganic trace elements in the diet of finishing pigs with organic trace elements of iron, copper, manganese and zinc (in the form of protein salts) significantly increased the zinc content in the serum of early pigs, but had no significant effect on the iron, copper and manganese contents [26]. In this experiment, replacing all inorganic trace elements with 75% or 100% oligosaccharide chelated trace element premix significantly increased the iron content in liver and serum of broiler feed. When replacing all inorganic trace elements with 50% oligosaccharide chelated trace element premix, there was no significant difference in the content of iron, copper, manganese and zinc in liver and serum of broiler feed, indicating that 50% oligosaccharide chelated trace elements can completely replace all inorganic trace elements. The reason may be that after the trace elements are chelated with oligosaccharides, stable chelates are formed, which reduces the antagonism between trace elements [27-29]; at the same time, the biological utilization rate of oligosaccharide chelated multi-trace elements is high, and the chelated ring can reduce the binding and deposition of trace elements by phytate and other precipitants in the intestine, and can be easily recognized and captured by protein receptors in the intestine, enter the blood circulation through the absorption pathway of small peptides and amino acids, and enter the liver for deposition, thereby increasing the content of trace elements in blood and liver [22,30-32].
3.3 Antioxidant enzymes and malondialdehyde content GSH-Px and Cu-Zn SOD constitute important barriers for the body to resist oxidative stress [33], and the MDA content reflects the body's degree of oxidation. CERONE et al. [34] found that the lack of copper and zinc would affect the activity of Cu-Zn SOD enzyme. Xue Ying et al. [35] confirmed that the addition of 25% and 50% of the NRC required amount of hydroxyamino acids manganese, copper, zinc, glycine iron, and yeast selenium to the feed significantly increased the activity of plasma SOD and GSH-Px and reduced the MDA content. Adding 120 mg/kg of glycine ferrous iron to the feed can significantly increase the SOD activity in broiler serum and reduce the MDA content [36]. Replacing the corresponding inorganic trace elements in the feed of finishing pigs with the organic trace elements iron, copper, manganese and zinc (in the form of protein salts) can increase the Mn-SOD activity of early pigs, reduce the MDA content, and significantly increase the GSH-Px activity of late pigs [26]. In this experiment, with the decrease of the level of oligosaccharide chelated multi-trace elements, the activities of Cu-Zn SOD and GSH-Px in broiler serum and liver decreased in a linear manner, while the content of MDA increased in a linear manner. When 75% of the inorganic trace elements were replaced by oligodisaccharide chelated trace element premix in broiler feed, the antioxidant capacity of broiler serum and liver was significantly improved. The antioxidant capacity of 75% of the oligodisaccharide chelated trace element premix replacing all inorganic trace elements was quite comparable to that of the inorganic trace element group. This may be because Cu-Zn SOD, as a copper and zinc dependent enzyme, is affected by the copper and zinc content in the blood and liver. At the same time, its increased activity may also be related to the improvement of its own degradation stability or the slowdown of its degradation rate. As the oligodisaccharide chelated multi-trace elements are absorbed by the body, the effective copper and zinc content in the blood and liver increases, and the Cu-Zn SOD activity also increases significantly, which improves the body's antioxidant enzyme activity and the ability to scavenge free radicals in the body [37-41]. At the same time, the increase in oligosaccharide content in the body also has the ability to scavenge free radicals in the body and improve the body's antioxidant enzyme activity [42-44]. The antioxidant enzyme activity of serum and liver at 21 days was lower than that at 42 days, which is related to the growth and development of animals, and also better explains one of the reasons why the resistance is strong with age.
Compared with the treatment of inorganic trace elements and the treatment without trace elements, 100% oligosaccharide chelated trace element premix can improve the growth performance, trace element content and antioxidant performance of broilers, and can improve the production performance of broilers, the reserve of elements (copper, zinc, manganese and iron) in serum and liver, and the optimal levels of serum and liver antioxidant performance are 25%, 50% and 75% respectively; the level equivalent to the comprehensive performance of inorganic trace elements is 50%-75%. As the addition level of oligosaccharide chelated trace element premix decreases, most indicators of broiler production performance, trace element content in blood and liver, and antioxidant enzyme activity decrease linearly.
References:
[1]RICHARDS JD, ZHAO JM, HARRELL RJ, et al. Trace mineral nutrition in poultry and swine[J]. Asian-Australasian Journal of Animal Sciences 2010, 23(11): 1527-1534.
[2]TIAN Jia, LIU Guohua, CAI Huiyi, et al. New progress in the research and application of trace elements in livestock and poultry[J]. China Poultry, 2016, 38(2): 37-41.
[3]ZHAO J, SHIRLEY R B, VAZQUEZ-ANON M, et al. Effects of chelated trace minerals on growth performance, breast meat yield, and footpad health in commercial meat broilers[J]. The Journal of Applied Poultry Research, 2010, 19(4): 365-372.
[4] Wang Lin, Wang Haihong, Han Xiangmin. Application of organic trace elements in chicken production [J]. Chinese Agricultural Science Bulletin, 2017, 33(08): 135-139.
[5] Wang Xiaolong, Huang Yiqiang, Chen Juan, Wang Shangchu. Advantages of organic trace elements [J]. Feed Wide Angle, 2018(08): 29-31.
[6] LU L, LIAO X D, LUO X G. Nutritional strategies for reducing nitrogen, phosphorus and trace mineral excretions of livestock and poultry [J] Journal of Integrative Agriculture, 2017, 16(12): 2815-2833.
[7] Niu Xianxiu, Yang Weiren, Cheng Zhenfeng, et al. Research progress on trace element supplementation levels in pig production[J]. Chinese Animal Husbandry and Veterinary Abstracts, 2018, 34(04): 244-246.
[8] WANG C, WANG M, YES S S, et al. Effects of copper-loaded chitosan nanoparticles on growth and immunity in broilers[J]. Poultry Science, 2011, 90(10): 2223-2228.
[9] Deng Zaofu. Effects of different amounts of methionine chelated zinc on the production performance of broiler chickens [J]. Animal Husbandry, 2006(02): 20-21.
[10] Wang Bo. Effects of oligosaccharide complexed trace elements on growth performance, feed nutrient metabolism rate and blood biochemical indexes of yellow-feathered broilers [D] Master's degree thesis, Nanning, Guangxi University, 2017.
[11] Lu Juanjuan, Cui Zhengan, Xia Zhongsheng, et al. Research on the replacement of inorganic trace elements with amino acid trace element chelates for feeding yellow-feathered broilers [J]. Feed and Animal Husbandry, 2011(07): 9-13.
[12] WANG Z, YU H, WUX, et al. Effects of Dietary Zinc Pectin Oligosaccharides Chelate Supplementation on Growth Performance, Nutrient Digestibility and Tissue Zinc Concentrations of Broilers[J]. Biological Trace Element Research,2016,173(2):475-482.
[13]Guo Jianlai,Li Mengyun,Zhu Kuanyou,et al. Effects of adding different proportions of compound organic trace elements on production performance, nutrient utilization and serum biochemical indices of piglets[J]. Feed Industry,2014,35(19):18-21.
[14]CREECH BL,SPEARS JW,FLOWERS WL,et al. Effect of dietary trace mineral concentration and source (inorganic vs. chelated) on performance,mineral status,and fecal mineral excretion in pigs from weaning through finishing[J]. Journal of Animal Science,2004,82(7):2140.
[15]Wang Zhuo. Comparative study of chitosan oligosaccharide and its chelated copper on animal copper absorption [D]. Liaoning Normal University, 2009.
[16] Deng Jihui, Yang Qiong, Shi Lingxiao. Research on the absorption mechanism of organic trace elements in livestock and poultry [J]. Animal Husbandry and Poultry Industry, 2012(03): 38-41.
[17] Ji Feng, Luo Xugang, Li Sufen, et al. Progress in animal organic trace element absorption mechanism and absorption research methods [J]. Journal of Animal Nutrition, 2003(02): 1-5+10.
[18] ANDRIEU S. Is there a role for organic trace element supplements in transition cow health? [J]. Veterinary Journal, 2008, 176(1): 77-83.
[19] HILL G M, MAHAN D C, JOLLIFF J S. Comparison of organic and inor-ganic zinc sources to maximize growth and meet the zinc needs of the nursery pig[J]. J. Anim. Sci, 2014, 92: 1582-1594.
[20] GOWANLOCK D W, MAHAN DC, JOLLIFF JS, et al. Evaluating the NRC levels of Cu, Fe, Mn, and Zn using organic minerals for grower-finisher swine[J]. Journal of Animal Science, 2013, 91(12).
[21] JIN Yaqian, LIU Wenzhong, REN Youshe, et al. Study on the distribution pattern and net requirement parameters of copper, iron, manganese and zinc in 35-50kg Dorper × Jinzhong sheep lambs[J]. Journal of Animal Science and Veterinary Medicine, 2016, 47(12): 2430-2440.
[22] LIU Jingbo. Effects of dietary trace element supplementation patterns on growth performance, meat quality, and trace element deposition and excretion of growing and finishing pigs[D]. Sichuan Agricultural University, 2018.
[23] Sun Qiujuan, Wang Yuming, Zhang Tianguo, et al. Effects of hydroxymethionine chelated copper/manganese/zinc on egg shell quality, enzyme activity and trace element deposition in laying eggs[J]. Journal of China Agricultural University, 2011, 16(04):127-133.
[24] SEO S H, LEE H K, LEE W S, et al. The Effect of Level and Period of Fe-methionine Chelate Supplementation on the Iron Content of Boiler Meat[J]. Asian Australasian Journal of Animal Science, 2011, 16(04):127-133. Science, 2008, 21(10).
[25] Li Zhihui, Cao Juan, Chen Li. Effects of hydroxymethionine chelated copper on growth performance and tissue copper content of weaned piglets and finishing pigs [J]. China Feed, 2019(06): 37-41.
[26] Ma Lianxiang, Hou Chuanchuan, He Junna, et al. Effects of compound organic trace elements on growth performance, serum indicators and trace element reduction in finishing pigs [J]. Journal of Zhejiang University (Agriculture and Life Sciences Edition), 2018, 44(02): 181-189.
[27] Zeng Lingzhi, Sun Dawei, He Shenghong, et al. .Application of chitosan oligosaccharide trace element chelate in the field of breeding[J]. China Feed, 2018(11): 66-70.
[28] Liu Fenfen, Ni Hengjia, Huang Pan, et al. Repair effect of hydroxymethionine zinc on cadmium damage in weaned piglets[J]. Journal of Animal Science and Veterinary Medicine, 2018, 49(02): 318-326.
[29] Zhan Kang, Li Yan, Bao Wenbin, et al. Effect of complex amino acid complex iron and zinc on production performance and some blood biochemical indicators of fattening pigs[J]. Journal of Animal Science and Veterinary Medicine, 2014, 45(05): 769-774.
[30] VEUM T L, CARLSON M S, WU C W, et al.Copper proteinate in weanling pig diets for enhancing growth performance and reducing fecal copper excretion compared with copper sulfate[J].J Anim Sci, 2004, 82: 1062~1070.
[31] DE MARCO M, ZOON M V, MARGETYAL C, et al. Dietary administration of glycine complexed trace minerals can improve performance and slaughter yield in broilers and reduces mineral excretion[J]. Animal Feed Science and Technology, 2017, 232: 182-189.
[32]BAO Y M, CHOCT M.Trace mineral nutrition for broiler chickens and prospects of application of organically complexed trace minerals: a review[J].Animal Production Science, 2009, 49: 269–282.
[33]Nia A B, Van Schooten F J, Schilderman P A E L, et al.A multi-biomarker approach to study the effects of smoking on oxidative DNA damage and repair and antioxidant defense mechanisms[J].Carcinogenesis, 2001, 22.
[34]CERONE S I, SANSINANEA A S, STREITENBERGER S A, et al. Cytochrome c oxidase, Cu, Zn-superoxide dismutase, and ceruloplasmin activities in copper-deficient bovines[J]. Biological Trace Element Research, 2000, 73(3): 269-278.
[35]Xue Ying, Dong Xiaofang, Tong Jianming. Effects of different levels of inorganic and organic composite trace elements on the antioxidant capacity of laying hens' plasma[J]. Journal of Animal Nutrition, 2016, 28(07): 2122-2131.
[36]Feng Guoqiang, Wu Jing, Fang Cuilin, et al. Effects of ferrous glycine on production performance, immune function and antioxidant indexes of broiler chickens[J]. Chinese Journal of Animal Husbandry, 2012, 48(11): 42-46.
[37]YUAN Y, JIN M, XIONG J, et al. Effects of dietary dosage forms of copper supplementation on growth, antioxidant capacity, innate immunity enzyme activities and gene expressions for juvenile Litopenaeus vannamei[J]Fish and Shellfish Immunology, 2019, 84: 1059-1067.
[38] MA Y F, HUANG Q C, LV M Y, et al. Chitosan-Zn chelate increases antioxidant enzyme activity and improves immune function in weaned piglets [J]. Biological Trace Element Research, 2014, 158(1): 45~50.
[39]WANG K K, WEI C H
Next Page
Next Page
RELATED PRODUCTS






